Currently, no CAR-T product has been approved for use in China despite having hundreds of active CAR-T trials. Relma-cel (JWCAR029) is a CAR-T product with a CAR construct comprised of a binding domain, derived from a murine CD19-specific mAb (FMC63), a CD3ζ activation domain, and a 4-1BB costimulatory domain, and manufactured in China with a commercial-ready, highly automated, single train process to select, activate, transduce and expand both CD4 and CD8 cells to a wide range of doses with consistent product attributes. Relma-cel was evaluated in the first prospective, single-arm, multi-center, pivotal study (RELIANCE Trial) of CAR-T therapy conducted under Chinese IND to support an NMPA-accepted BLA submission in r/r large B-cell lymphoma (LBCL) (NCT 04089215) and the preliminary results are presented herein.
Eligible adult patients had measurable, histologically confirmed r/r LBCL, failed at least 2 prior therapies, including anthracycline and anti-CD20 agents, and not had allogeneic transplant within 90 days or primary CNS lymphoma. Patients were randomized to receive either 100×106 (low dose) or 150×106 (high dose) CAR+ T cells as a single infusion following fludarabine 25 mg/m2 & cyclophosphamide 250 mg/m2 daily×3 as lymphodepletion. Patients were evaluated for efficacy (Lugano criteria, 2014), safety (CRS by Lee 2014, and all other events by CTCAE v4.03), and PK (by qPCR and flow cytometry). The protocol pre-specified and regulatory authority-agreed primary endpoint was 3 month ORR by landmark analysis to exclude a 3-month 20% ORR (Michael, 2017). Secondary endpoints included best overall response rate (BOR), duration of response (DOR), progression free survival (PFS), overall survival (OS), frequency/severity of AEs and CAR-T cell expansion. Investigators adjudicated disease response and progression based on imaging studies, pathology and clinical data; a sensitivity analysis was also conducted using an independent review committee adjudication.
Between May 2018 and Dec 2019, 69 patients were screened, and 59 enrolled and treated with relma-cel, with median age of 56.0 years (18-75), 59.3% ECOG 1-2, and included several LBCL subtypes (DLBCL NOS 69.5%, TFL 15.3%, DHL/THL 5.1%, and PMBCL 6.8%) and nearly all with poor risk disease (81.4% treatment refractory, 45.8% ≥3 lines of prior therapies, 42.4% requiring bridging therapy, 39% IPI≥3). The median doses administered in low and high dose groups were 99.7×106 (range, 80.1-101.3) and 150.0×106 (range, 120.0-156.4) CAR+ T cells, respectively. Among the 58 efficacy evaluable patients, the primary analysis demonstrated a 3 month ORR of 58.6% (95% CI, 44.9-71.4) (the excluded patient had product that did not meet viability threshold criterion, but achieved CR at D29 that is ongoing for >1 year). As of Jun 17, 2020 data cut-off, BOR was 75.9% (95% CI, 62.8-86.1) with CR rate of 51.7% (95% CI, 38.2-65.1). With a median follow-up of 8.9 months, median OS were not reached, and 6 month DOR, PFS and OS were 60.0%, 54.2% and 90.8%, respectively. No improvement in efficacy outcomes was observed in the high dose group.
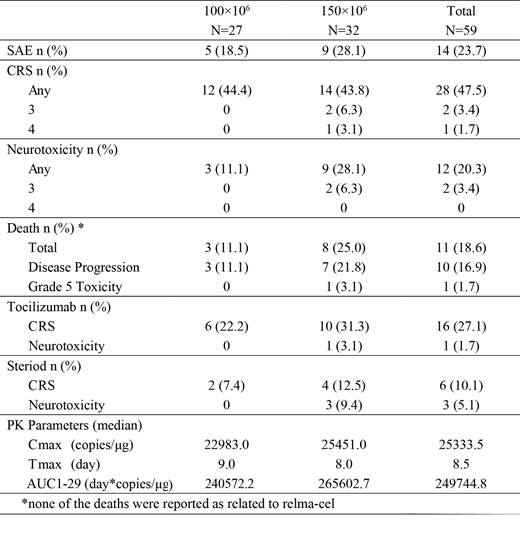
Of 59 treated patients, grade ≥3 AEs occurring in >5% of patients were cytokine release syndrome (CRS), febrile neutropenia and lung infection (all, 5.1%). Grade ≥3 lab abnormalities in >10% of patients were neutrophil count decreased (30.5%), white blood cell count decreased (13.6%), leukopenia (10.2%) and neutropenia (10.2%). The rates of CRS, neurotoxicity (NT), death, and the use of tocilizumab/steroids are shown in Table 1. All events of CRS and NT resolved except for 1 patient with grade 4 CRS ongoing at the time of death as the result of sepsis at day 8. As of data cut off, no cases of severe cytopenia or severe infections occurred beyond 30 days of infusion. The two dose groups did not differ significantly in PK parameters (see Table 1).
The RELIANCE Trial provided the first demonstration of licensure-quality CAR-T manufacturing and clinical trial data generation in r/r patients originating in China. These results with relma-cel demonstrate similar preliminary response rates and PK profiles while providing the potential for an improved toxicity profile in heavily-pre-treated patients with r/r DLBCL having poor risk features relative to other CD19-specific CAR-Ts approved in the US and EU.
Zhang:JW Therapeutics (Shanghai) Co. Ltd: Current Employment, Current equity holder in private company. Yang:JW Therapeutics (Shanghai) Co. Ltd: Current Employment, Current equity holder in private company. Zhou:JW Therapeutics (Shanghai) Co. Ltd: Current Employment, Current equity holder in private company. Zheng:JW Therapeutics (Shanghai) Co. Ltd: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal